
Farm Tours
We offer farm tours for smaller groups and families to meet, feed, interact with and walk with our alpacas.
For full details and to book your experience of these wonderful animals, please go to our reservation form.
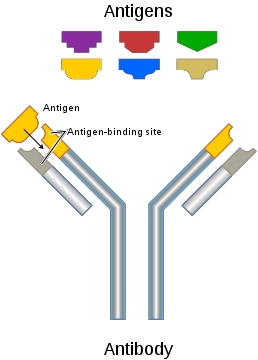
The two heavy and two light chains self-assemble into a 'Y'-shaped structure, as shown in the image. The stem of the Y (called the Fc region) is relatively conserved in structure and interacts with certain components of the immune system. The tips of the Y arms (Fab regions) contain highly variable loops known as the complementarity-determining regions (CDRs) that determine the specific antigen each antibody binds to.
Conventional antibodies are large molecules, approximately 150 kilodaltons (kDa) in mass and about 10 nm in length. They belong to five main classes - IgA, IgD, IgE, IgG, and IgM, each with specific roles in the immune process. IgA protects mucosal surfaces, IgE is involved in allergy and parasite defence and IgG, which is the most abundant antibody type in blood plasma, provides systemic protection.
Camelids produce an additional type of antibody and about 40% of circulating antibodies are “heavy-chain only antibodies” (HCAbs). These lack light chains entirely and are composed of just two heavy chains [27]. Despite their simplified structure, they retain full antigen-binding capacity. The functional binding portion of these heavy-chain antibodies is contained in a single variable domain, called VHH. When isolated, this domain is called a nanobody [27]. Nanobodies are small (between 12 and 15 kDa), highly stable and capable of binding to hidden or recessed epitopes, inaccessible to conventional antibodies. These unique properties have made nanobodies valuable tools in biotechnology, diagnostics, and therapeutic development.
| Feature | Conventional Antibody (IgG) | Nanobody (VHH) |
|---|---|---|
| Molecular size | ~150 kDa; ~10 nm | around 12 to 15 kDa; much smaller |
| Structure | Y-shaped: 2 heavy + 2 light chains | Single variable domain only |
| Binding site access | Good for exposed epitopes | Excellent for recessed/cryptic epitopes |
| Stability | Moderate; sensitive to heat & pH | High thermal & chemical stability |
| Solubility | Lower at high concentration | Very soluble; high-concentration formulations easier |
| Production | Mammalian cell culture (e.g., CHO) | Microbial (E. coli/yeast) or mammalian; cheaper/faster |
| Tissue penetration | Limited (large size) | Excellent (small size) |
| Half-life | Long (days-weeks) | Short unless engineered (e.g., Fc/albumin fusion) |
| Delivery options | Primarily IV/SC | IV/SC; inhaled, topical, intranasal explored |
| Immunogenicity | Well understood; humanisation common | Low–moderate; humanisation often used |
The Coronavirus family causes a wide range of illnesses in animals and humans, from the common cold to severe conditions such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). In camelids, a coronavirus has been linked to diarrhoea in young crias [28, 29]. These viruses can be zoonotic, with wildlife acting as reservoirs [31][32].
SARS-CoV-2 (COVID-19) is thought to have originated in bats, possibly transmitting to humans through pangolins. It has been shown to infect numerous non-human species - including cats, dogs, great apes, big cats, mink, and hippopotamus, but is unlikely to spill over into livestock such as cattle, sheep, goats, rabbits, horses, or alpacas [58–64].
A key step in SARS-CoV-2 infection is binding of the viral spike glycoprotein to the ACE2 receptor. Nanobodies raised against the spike protein block this process by binding tightly to the spike [33], preventing viral entry. Their small size, solubility, and stability make them attractive as therapeutic agents, including in inhalable formulations targeting the lungs [35]. Structural studies (e.g., synchrotron analysis) have mapped nanobody–spike interactions to identify precise binding regions.
A 2021 accelerated Nature study [51] showed that nanobodies raised in llamas, and in mice engineered to produce camelid nanobodies, remained effective despite antigenic drift. They also recognised epitopes inaccessible to human antibodies, supporting their therapeutic value against emerging variants. Another receptor, Neuropilin-1, abundant in the nasal cavity, has been shown to enhance SARS-CoV-2 infectivity. Blocking it with antibodies reduced viral entry, making it a potential target for nanobody development [46].
Beyond therapeutics, camelid nanobodies have been used to develop an electrochemical COVID-19 test. Reported in The Guardian (23/2/2021), the test is three times faster than lateral flow assays and almost as accurate as PCR.
Snakebite envenomation affects ~2.7 million people annually, causing ~110,000 deaths and leaving many survivors with amputations, kidney failure or a permanent disability [53]. Conventional antivenoms are produced by immunising horses or sheep with a venom, then purifying the resulting antibodies from the blood. Camelids offer an alternative to this. Alpaca-derived nanobody antivenoms have been developed in Australia against local snakes, some of the most toxic in the world [54]. Peruvian researchers have raised llama nanobodies against the fer-de-lance (Bothrops atrox), a major cause of fatalities in South America [55]. These camelid antivenoms demonstrated comparable or superior activity to conventional sera and successfully treated cases such as a dog bitten by a tiger snake [56]. Nanobodies provide an additional advantage by binding toxin epitopes inaccessible to traditional antibodies.
Beyond COVID-19 and snakebite treatment, nanobodies have shown promise in a variety of biomedical contexts including:
Most of the literature below can be accessed by clicking on the highlighted link. Some links will access the appropriate web page from which the article can be downloaded but others will immediately start downloading the full reference.
27. Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hamers, C.,Songa, E.B., Bendahman, N., Hamers, R. (1993). Naturally occurring antibodies devoid of light chains. Nature, 363: 446–448.
28. Cebra, C.K., Mattson, D.E., Baker, R.J., Sonn, R.J., Dearing, P.L. (2003). Potential pathogens in feces from unweaned llamas and alpacas with diarrhea. J. Am. Vet. Med. Assoc. 223(12): 1806–1808.
29. Jin, L, Cebra, C.K., Baker, R.J., Mattson, D.E., Cohen, S.A., Alvarado, D.E. and Rohrmann, G.F. (2007). Analysis of the genome sequence of an alpaca coronavirus. Virol., 365(1): 198-203.
30. Vanlandschoot, P., Stortelers, C., Beirnaert, E., Ibañez, L.I., Schepens, B., Depla, E., Saelens, X. (2011). Nanobodies: new ammunition to battle viruses. Antivir. Res., 92: 389-407.
31. Poon, L.L.M., Chu, D.K.W., Chan, K.H., Wong, O.K., Ellis, T.M., Leung, Y.H.C., Lau, S.K.P., Woo, P.C.Y., Suen, K.Y., Yuen, K.Y., Guan, Y. and Peiris, J.S.M. (2005). Identification of a Novel Coronavirus in Bats. J. Virol., 79(4): 2001–2009.
32. Lam, T.T., Jia, N., Zhang, Y. et al. (2020). Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature, 583: 282–285.
33. Hanke, L., Perez, L.V., Sheward, D.J., Das, H., Schulte, T., Moliner-Morro, A., Corcoran, M., Achour, A., Karlsson Hedestam, G.B. Hällberg, B.M., Murrell, B. and McInerney, G.M. (2020). An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. Posted 8th July 2020. doi: https://doi.org/10.1101/2020.06.02.130161.
35. Gai, J., Ma, L., Li, G., Zhu, M., Qiao, P., Li, X., Zhang, H., Zhang, Y., Chen, Y., Gong, R. and Wan, Y. (2020). A potent neutralizing nanobody against SARS-CoV-2 with inhaled delivery potential. BioRxiv Preprint server.
36. Jovčevska, I. and Muyldermans, S. (2020). The Therapeutic Potential of Nanobodies. BioDrugs, 34: 11-26.
37. Steeland, S., Vandenbroucke, R.E. and Libert, C. (2016). Nanobodies as therapeutics: big opportunities for small antibodies. Drug Disc. Today, 21(7): 1076-1113.
38. Lin, J., Gu, Y., Xu, Y., Yu, J., Tang, J., Wu, L., Zhou, Z., Chen, C., Liu, M., Chun, X., Liu, H., Nian, R., Song, H. and Zhang, J. (2020). Characterization and applications of nanobodies against Pseudomonas aeruginosa Exotoxin A selected from single alpaca B cells. Biotech. Biotechnol. Equip., 34(1): 1028–1037.
39. Chames, P. and Rothbauer, U. (2020). Special Issue: Nanobody. Antibodies 2020, 9(1), 6; https://doi.org/10.3390/antib9010006.
40. Ji, L., Dong, C., Fan, R. and Qi, S. (2020). A high affinity nanobody against endothelin receptor type B: a new approach to the treatment of melanoma. Mol. Biol. Rep., 47, 2137-2147.
41. Xia, L., Teng, Q., Chen, Q. and Zhang, F. (2020). Preparation and Characterization of Anti-GPC3 Nanobody Against Hepatocellular Carcinoma. Int. J. Nanomed., 15: 2197-2205.
42. Dumoulin, M., Last, A.M., Desmyter, A., Decanniere, K., Canet, D., Larsson, G., Spencer, A., Archer, D.B., Sasse, J., Muyldermans, S., Wyns, L., Redfield, C., Matagne, A., Robinson, C.V. and Dobson, C.M. (2003). A camelid antibody fragment inhibits the formation of amyloid fibrils byhuman lysozyme. Nature, 424: 783-788.
43. He, Y., Ren, Y., Guo, B., Yang, Y., Ji, Y., Zhang, D., Wang, J., Wang, Y. and Wang, H. (2020). Development of a specific nanobody and its application in rapid and selective determination of Salmonella enteritidis in milk. Food Chem., 310, 25 April 2020, 125942.
44. Chames, P. and Rothbauer, U. (2020). Special Issue: Nanobody. Antibodies, 9(1): 6-9.
45. Schumacher, D., Helma, J., Schneider, A.F.L., Leonhardt, H. and Hackenberger, C.P.R. (2018). Nanobodies: Chemical Functionalization Strategies and Intracellular Applications. Angew. Chem. Int. Ed., 57: 2314-2333.
46. Cantuti-Castelvetri, L., Ojha, R., Pedro, L. D., Djannatian, M., Franz, J., Kuivanen, S., van der Meer, F., Kallio, K., Kaya, T., Anastasina, M., Smura, T., Levanov, L., Szirovicza, L., Tobi, A., Kallio-Kokko, H., Österlund, P., Joensuu, M., Meunier, F. A., Butcher, S. J., Winkler, M.S., Mollenhauer, B., Helenius, A., Gokce, O., Teesalu, T., Hepojoki, J., Vapalahti, O., Stadelmann, C., Balistreri, G. and Simons, M. (2020). Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Sci., 20 Oct.
48. Yang E. Y., Shah K. (2020). Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front. Oncol., 23 July.
51. Xu, J., Xu, K., Jung, S. et al. (2021). Nanobodies from camelid mice and llamas neutralize SARS-CoV-2 variants. Nature. https://doi.org/10.1038/s41586-021-03676-z.
53. World Health Organisation (2021). Snakebite envenoming. Available online. Accessed 20/10/2021.
54. Padula, A. M. and Winkel, K.D. (2017). Antivenom production in the alpaca (Vicugna pacos): Monovalent and polyvalent antivenom neutralisation of lethal and procoagulant toxins in Australian elapid venoms. Small Ruminant Res., 149: 34-39.
55. Calderon, H.B., Coronel1, V.O.Y., Rey, O.A.C., Alave1, E.G.C., Duran, W.J.L., Rojas, C.P., Arevalo, H.M., Neyra, D.G., Pérez, M.G., Bonilla, C., Tintaya, B., Ricciardi, G., Smiejkowska, N., Romão, E., Vincke, C., Lévano, J., Celys, M., Lomonte, B. and Muyldermans, S. (2020). Development of Nanobodies Against Hemorrhagic and Myotoxic Components of Bothrops atrox Snake Venom. Front. Immunol., 11: 1-12.
56. Padula, A. M. and Winkel, K.D. (2016). Successful use of camelid (alpaca) antivenom to treat a potentially lethal tiger snake (Notechis scutatus) envenomation in a dog. Toxicon, 114: 59-64.
58. Bosco-Lauth, A.M., Walker, A., Guilbert, L., Porter, S., Hartwig, A., McVicker, E., Bielefeldt-Ohmann, H. and Bowen, R.A. (2021). Susceptibility of livestock to SARS-CoV-2 infectionEmerging Microbes and Infections, 10: 2199-2201.
59. Calvet, G.A., Pereira, S.A., Ogrzewalska, M., Pauvolid-Corrêa, A., Resende, P.C., Tassinari, W. de S., de Pina Costa,A., Keidel, L.A., et al. (2021) Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS ONE 16(4): e0250853. https://doi.org/10.1371/journal.pone.0250853.
60. UNESCO (2021). Gorillas test positive to COVID-19: what it means for Great Apes. Web article, 12th January 2021.
61. France 24 report (2021). Lions at Singapore wildlife park infected with coronavirus. Web article, 10th November 2021.
62. Smithsonian’s National Zoo & Conservation Biology Institute (2021). Great Cats Tested Presumptive Positive For COVID-19 at the Smithsonian's National Zoo. Web article, 17th September 2021.
63. Chaintoutis SC, Thomou Z, Mouchtaropoulou E, Tsiolas G, Chassalevris T, Stylianaki I, et al. (2021). Outbreaks of SARS-CoV-2 in naturally infected mink farms: Impact, transmission dynamics, genetic patterns, and environmental contamination. PLoS Pathog., 17(9): e1009883.
64. BBC News (2021). Belgian zoo hippos test positive for Covid. Web article, 4th December 2021.
66. Dulal, H.P., Vance, D.J., Neupane, D.P., Chen, X., Tremblay, J.M., Shoemaker, C.B., Mantis, N.J. and Song, J. (2022). Neutralization of typhoid toxin by alpaca-derived, single-domain antibodies targeting the PltB and CdtB subunits. Infect. Immun., 90(2): Feb.
70. Li, Q., Zhang, F., Lu, Y., Hu, H., Wang, J., Guo, C., Deng, Q., Liao, C., Wu, Q., Hu, T., Chen, Z. and Lu, J. (2022). Highly Potent Multi-Valent Nanobodies Against Chikungunya with VHH Screened from Alpaca Naïve Phage Display Library. DOI: Research Square pre-publication.
71. Panteleev, P.V., Safronova, V.N., Kruglikov, R.N., Bolosov, I.A., Bogdanov, I.V. and Ovchinnikova, T.V. (2022). A Novel Proline-Rich Cathelicidin from the Alpaca Vicugna pacos with Potency to Combat Antibiotic-Resistant Bacteria: Mechanism of Action and the Functional Role of the C-Terminal Region. Membranes, 12: 515-533.
72. Stokstad, E. (2023). Antibody-based defense may protect plants from disease. Sci., 379(6635): 867.
79. Qiu, J., Li, J., Zhang, Z., Dong, S., Ling, X., Fang, Z., Ling, Q. and Huang, Z. (2023). Construction of an alpaca immune antibody library for the selection of nanobodies against Drosophila melanogaster proteins. Front. Bioeng. Biotechnol. 11: 1207048.
83. Alexander, E. and Leong, K.W. (2024). Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years. J Nanobiotechnol 22: 661. https://doi.org/10.1186/s12951-024-02900-y.